Nettside ABFredriksen LinkedIn Facebook Vaccibody
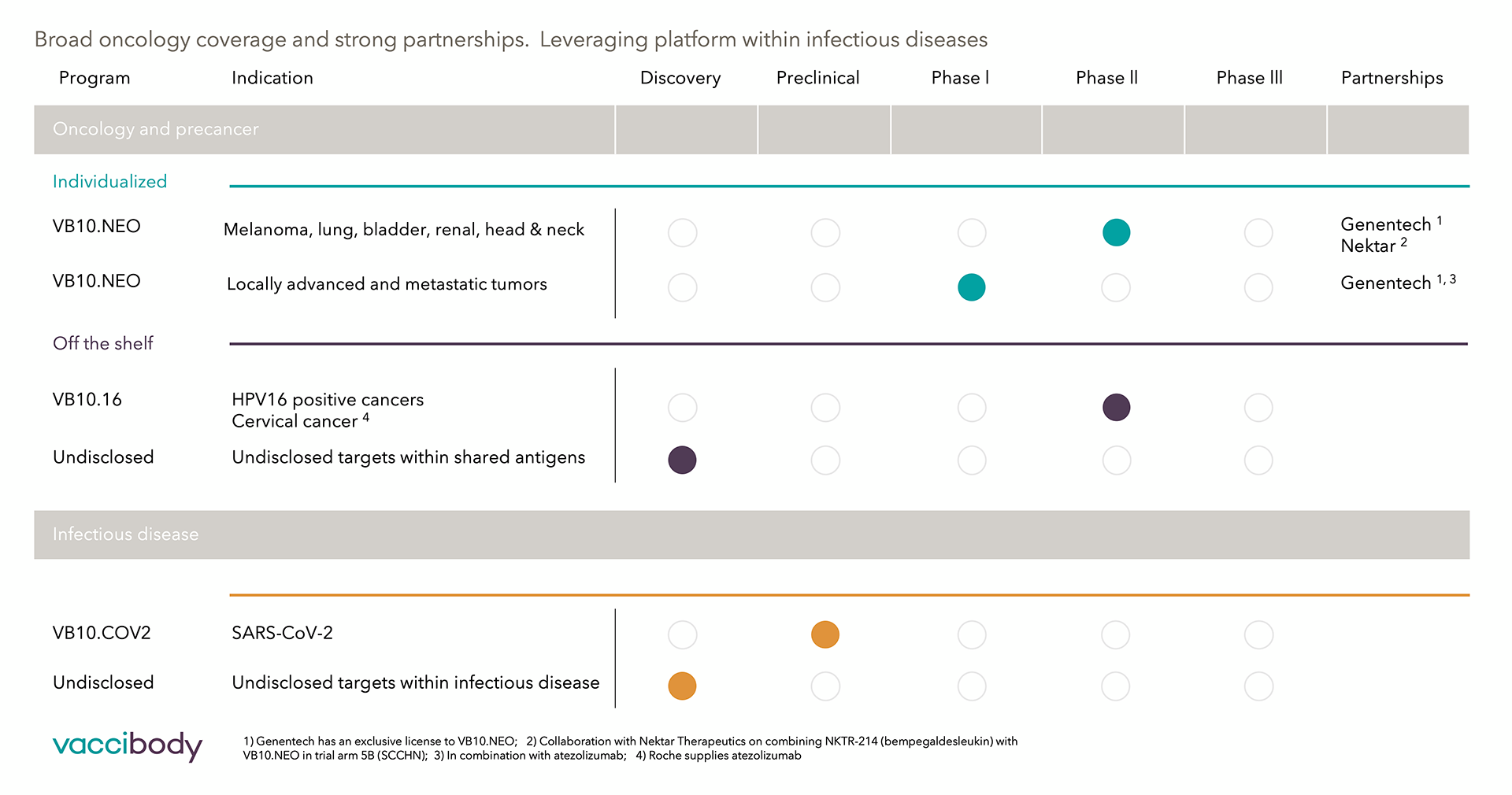
Our company was founded in 2007 and has since then, become one of the world’s most exciting Biotech companies, rapidly growing. Today we are counting more than 60 amazing employees within medical and development teams, manufacturing, bioinformatics, and research teamsWe use the Vaccibody technology to generate therapeutics with best-in-class potential to treat cancers with a high unmet medical need. Furthermore, we also focus our research on infectious diseases and immune tolerance. We closely collaborate with Roche and Nektar Therapeutics. And we are proud to have entered into a worldwide license and collaboration agreement with Genentech, a member of the Roche Group, to develop individualized neoantigen cancer vaccines.
Amazing opportunity to join Vaccibody, one of the world’s most exciting Biotech companies.

QC Scientist
✔Analytical testing & documentation ✔Lab work ✔SOPs ✔Chromatographic principles ✔Protocols & reports ✔Troubleshooting
At Vaccibody we pride ourselves on our passion for meaningful work and dedicated cross-functional teams. Our team continues to grow, and we are now recruiting for a highly motivated and enthusiastic QC Scientist – CMC to join our adventure in the further development of the Vaccibody vaccine platform.
Key responsibilities:
- Execution and documentation of analytical method development
- Coordination with external analysis labs
- Qualification and maintenance of analytical equipment
- External and internal collaboration
Education and experience requirements:
- MSc in Analytical Sciences or related field
- Experience within Analytical Sciences from Pharma or Biotech
- Laboratory work experience, especially within chromatographic principles (HPLC and/or CE) and general test methods for pharmaceutical products
- Setting up procedures and analysis methods with a focus on robustness
- PCR, ELISA, bioassays etc. is a plus, but not strictly necessary
- Writing SOPs for analytical methods and instruments
- Writing protocols and reports for analytical work
- Troubleshooting in analytical methods
- Work in orderly manner and be good at preparing documentation of analytical experiments
- Skilled in use of IT systems
- Proficiency in English both verbal and in writing
Other qualifications:
- Have strong interpersonal skills and possess the ability to communicate with other development staff in a fast-paced setting
- Take responsibility and be able to work independently
- Flexibility and enjoy handling multiple tasks at the same time
- Be a team player with a “we” mentality
- Have an analytical, structured, and regulatory mindset
Location:
Forskningsparken, Oslo, Norway
Employment Type:
Permanent. Full-Time
Application process:
This recruitment is carried out in collaboration with the recruitment company Borka Consulting AS. If you have any questions regarding Vaccibody or the position, feel free to contact our recruitment advisor in Borka Consulting; Cecilie Fraas Borka at +47 928 55 352 or André Borka at +47 908 31 871.
Please send your application (both motivational letter and CV in PDF format) to cecilie@borka.no. Norwegian applicants may apply in Norwegian. In the application letter, please highlight which of the above-mentioned “Education, experience and other qualifications » you fulfill. All inquiries are treated confidentially, also towards Vaccibody in the initial phase if desired.
Deadline for applications: July 16th.
Who are we looking for?
When hiring new employees, we look for the individuals who we think would be a good fit with our collaborative and inclusive company culture. You enjoy working together with other people in cross-functional team structures. Moreover, you appreciate working in an international environment.
Why join our Vaccibody team?
At Vaccibody, we offer a fun and dynamic international working environment. The people are at the heart of our organization, which is why we always keep striving to offer a workplace where we cheer each other on and find motivation. Your skills and competencies are unique and to ensure continued development our aspiration is to provide you with good opportunities for personal growth. We care about each other.
About us:
Our company was founded in 2007 and has since then, become one of the world’s most exciting Biotech companies, rapidly growing. Today we are counting more than 60 amazing employees within medical and development teams, manufacturing, bioinformatics, and research teamsWe use the Vaccibody technology to generate therapeutics with best-in-class potential to treat cancers with a high unmet medical need. Furthermore, we also focus our research on infectious diseases and immune tolerance. We closely collaborate with Roche and Nektar Therapeutics. And we are proud to have entered into a worldwide license and collaboration agreement with Genentech, a member of the Roche Group, to develop individualized neoantigen cancer vaccines.

Biotek, Biotech, individualized cancer neoantigen vaccines, Oncology, Onkologi, immunotherapies, infectious diseases, DNA vaccines, cervical cancer, Kreft, Kreftvaksine, Covid, Euronext Growth Oslo, biopharmaceutical, Genentech, Roche, Nektar Therapeutics, Oslo Science Park, Forskningsparken