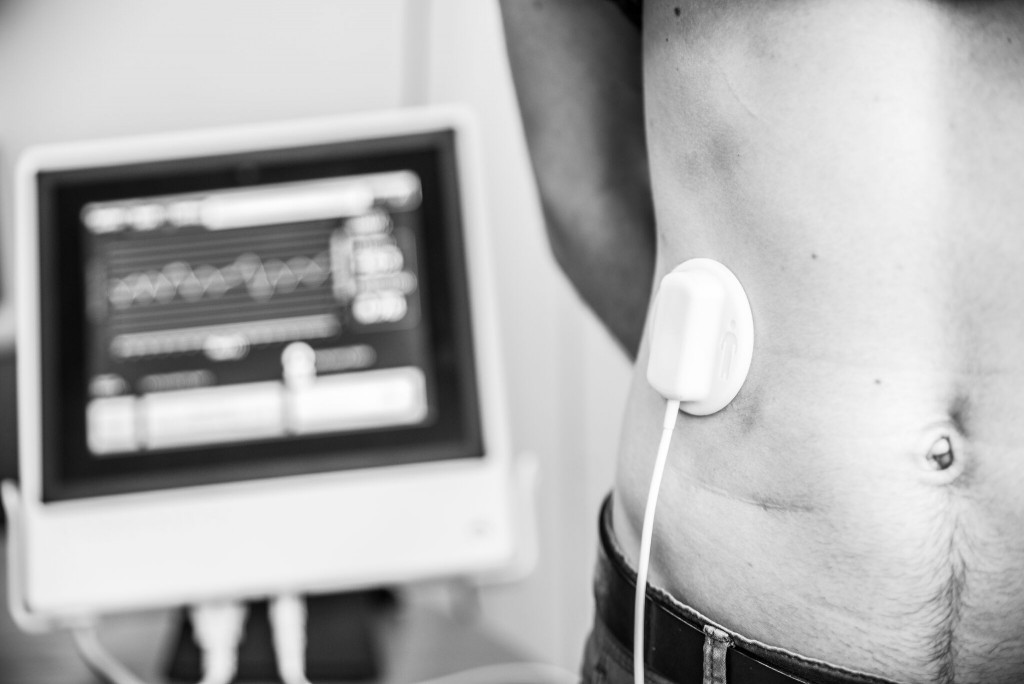
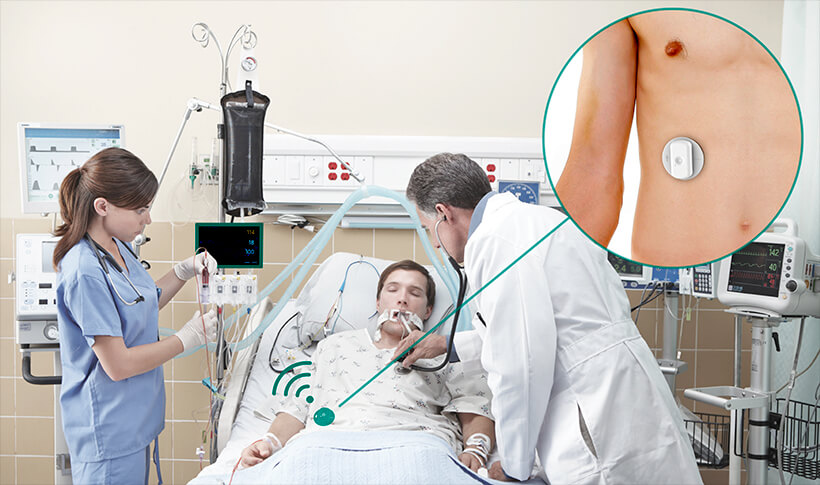
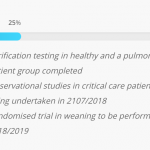
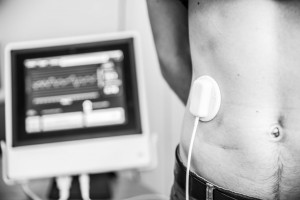
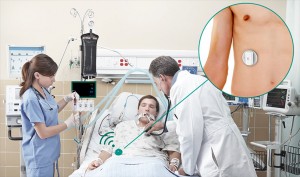
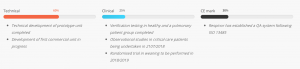
Respinor AS is a Norwegian medical device company founded in 2015 and was in 2017 ranked number one among 237 companies in Europe by the Horizon 2020. Respinor is developing a novel non-invasive medical device for continuous monitoring of the diaphragm, the main breathing muscle. Currently, the company has initiated clinical studies in Europe for the first indication mechanical ventilation (MV) and estimate to launch in EU by the end of 2020 and expand into other key markets globally. Our technology has a broad range of potential benefits and represents an enabling technology platform for applications in critical care and other areas of respiratory medicine. The company aim at providing an objective measure of breathing addressing a global market with a cutting-edge medical technology. Because every breath counts. See more at: www.respinor.com
Regulatory & Quality Director
Med Tech – International experience – Enterprising environment
Respinor is rapidly growing and is seeking a Regulatory and Quality Director to join its team of dedicated professionals. The company aim at providing an objective measure of breathing addressing a global market with a cutting edge medical technology. Because every breath counts.
To prepare for market introduction we seek a Regulatory and Quality Director who will be responsible of the day to day management and operation of the Regulatory requirements and Quality System.
Specific responsibilities include:
- Follow up Technical file towards CE approval.
- Prepare and write 510(k) submissions and Letters-To-File.
- Act as the regulatory representative in the product development teams and the management team.
- Develop regulatory strategy for our technology and devices Globally with main focus on the EU and U.S.
- Reviews device changes to assess regulatory impact.
- Gather information and data from other departments and external resources for regulatory submissions in EU and the U.S.
- Review and approve advertising and promotional materials.
- Implementation and management of the quality system.
- Ensuring compliance with all applicable regulations concerning the manufacture of the company’s products.
- Preparation of regulatory filings in support of marketing authorizations and clearances.
- Management of critical vendors in product design, development, testing and manufacture.
- Release of Products.
Who are you?
The ideal candidate has at least 3 years in a regulatory and quality management role in an ISO13485 or QSR registered and compliant facility. Additionally, at least 1 years in a product development role in a medical product company. Preferably you have experience of leading inspections by Notified Bodies of medical device manufacturer and have managed technical files for FDA and CE mark submissions.
We can offer a vibrant and creative working environment in the Norway Health Tech cluster situated in Oslo Research Park (Forskningsparken). Candidates will be expected to have their residence in Oslo Area.
For more information and about the application process, please contact external recruiter André Borka in Borka Consulting at +47 90831871. Please send your motivational letter and CV in PDF format to andre@borka.no. All inquiries are treated confidentially, also towards Respinor in the initial phase if desired.