Nettside ABFredriksen Vaccibody
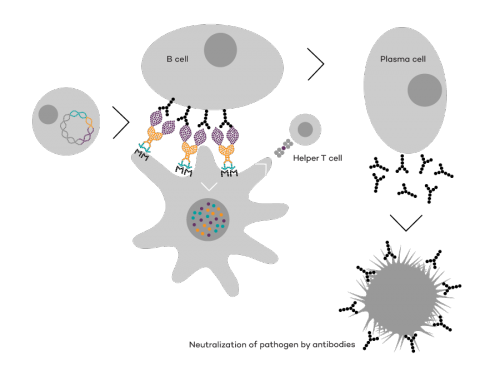
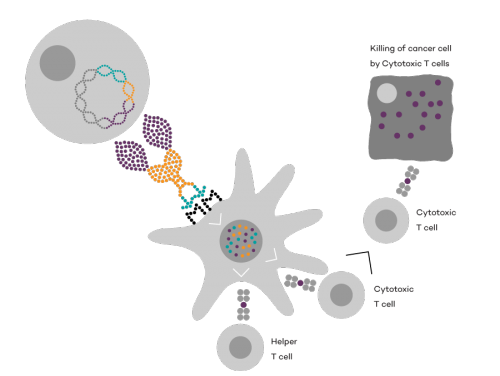
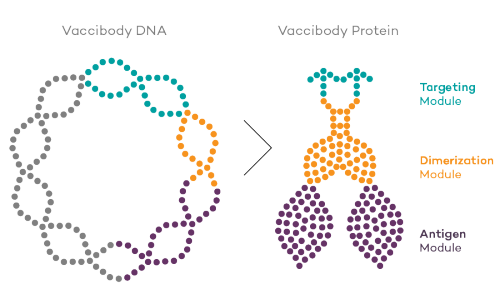
Vaccibody is a leading cancer neoantigen company, which bases it vaccine development on a proprietary patent protected vaccine technology allowing for generation of very efficacious, targeted and safe naked plasmid DNA vaccines. Vaccibody is looking to enter cancer neoantigen clinical trials within its VB10.NEO program (implies one cancer vaccine custom-made per patient). The company moreover has recently finalized a clinical phase I trial with VB10.16 (a therapeutic HPV vaccine) and plans to start clinical phase IIa in Q2, 2017
Head of CMC (Chemistry, Manufacturing and Controls)
Vaccibody is now looking for a Head of CMC. The CMC leader will report to the CEO and will be responsible for all CMC-related activities for both VB10.NEO and VB10.16 including process development, chemical and biological manufacturing, formulation and supply of clinical trial material. The ideal candidate will be a highly motivated individual with experience from and interest in working in a small, entrepreneurial environment with broad responsibilities and opportunities.
Principal Duties and Responsibilities:
- Overall responsibility for all drug substance and drug product activities from preclinical development through clinical supplies for clinical trials
- Develop and implement strategy and process for GMP manufacturing of neoantigen-based Vaccibody DNA plasmid cancer vaccines on demand
- Identification, selection and management of CMOs for process optimization, technology transfer, GMP manufacture and supply of drug substance (API) and drug product (DP).
- Hire and lead Vaccibody’s internal CMC team
- Develop and implement strategy for optimizing and controlling quality of bulk API and DP using CROs and CMOs
- Delivery of robust, scalable and cost-effective manufacturing routes.
- Implement stage appropriate analytical methods and protocols to ensure that all CROs and CMOs are using systems and processes in compliance with all relevant regulatory standards
- Writing and reviewing documents for regulatory submissions; represent the company as the CMC expert before European and US regulatory authorities
Skills and background:
- PhD or MSc in chemical engineering, pharmaceutical sciences, biochemistry, biology or related scientific discipline
- 10+ years of relevant experience in a pharmaceutical or biotech CMC environment
- Substantial experience with managing CROs/CMOs
- Experienced with GMP manufacturing and CTA, IND and other relevant filings, including knowledge of relevant EMA and FDA regulations
- Solution-oriented with strong ability to thrive while operating in the front of a new field with limited regulatory guidelines
- Strong ability to identify and resolve critical issues
- Excellent written and verbal communication skills, very strong interpersonal and management skills to collaborate with and direct the work of others (both internal colleagues and external collaborators).
For more information and about the application process please contact external recruiter:
Cecilie Borka, Borka Consulting at +47 92855352. Please send your application (motivational letter and CV) in PDF format to cecilie@borka.no. Interviews are held consecutively, so show your interest as soon as possible!
All inquiries are treated confidentially, also towards Vaccibody in the initial phase if desired
—
Se TV2-innslag her: http://www.tv2.no/v/927503/