Nettside ABFredriksen LinkedIn Facebook Vaccibody
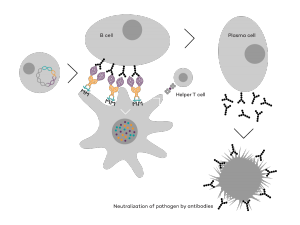
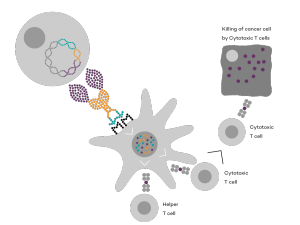
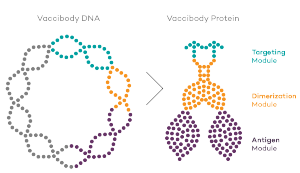
Vaccibody is an immunotherapy company dedicated to the discovery and development of novel immunotherapies. Vaccibody is a leader in the rapidly developing field of individualized cancer neoantigen vaccines and is using the Vaccibody technology to generate best-in-class therapeutics to treat cancers with a high unmet medical need. Vaccibody AS has developed a unique and innovative vaccine platform with the aim to treat and prevent pre-cancerous diseases or cancer as well as infectious diseases. Through its innovative design Vaccibody’s proprietary vaccine platform generates rapid, durable and broad antibody and T cell responses leading to remarkably potent vaccines
CMC Manager
Vaccibody is now looking for a CMC Manager to be responsible for CMC-related activities for both the VB10.NEO and VB10.16 programmes including process development and manufacturing of biologics, formulation, supply of clinical trial material and CMO/CRO oversight. The ideal candidate will be a highly motivated individual with experience from and interest in working in a small, entrepreneurial environment with broad responsibilities and opportunities.
Principal Duties and Responsibilities:
- Contribute to developing, implementing, maintaining and improving the GMP production of neoantigen-based DNA plasmid cancer vaccines
- Contribute to developing and implementing processes to optimize and control the quality of the bulk API (Active Pharmaceutical Ingredient) and DP (Drug Product) and discuss the strategies with the authorities
- Use risk assessment tools actively in the development work
- Write, update and quality assure relevant documents for submission to regulatory authorities
- Represent Vaccibody during audits of collaborators (CMO’s, CRO’s)
- Work closely with CMOs, CROs and other collaborators
- Support with the selection and establishment of new collaborators within production, analytical and regulatory work
The ideal candidate has:
- PhD or MSc in biology, biochemistry, pharmaceutical sciences, chemistry or related scientific discipline.
- expertise in biological production, and preferably knowledge of DNA plasmids
- several years of relevant experience in a biotech or pharmaceutical CMC environment
- experience with managing CROs/CMOs
- knowledge of GMP production, IMPD and IND, including knowledge of relevant EMA and FDA regulations
- ability to identify and solve critical problems
- ability to think out of the box and challenge current CMC development strategies
- fluent English language skills, both oral and written
The position will report to Mette Husbyn, CTO. The workplace is Forskningsparken in Oslo
For more information, please contact external recruiter:
Cecilie Borka, Borka Consulting at +47 92 85 53 52. Please send your application (both motivational letter and CV) in PDF format to cecilie@borka.no
Interviews are held consecutively, so show your interest as soon as possible! All inquiries are treated confidentially, also towards Vaccibody in the initial phase if desired
–
Nyheter: http://www.vaccibody.com/news/
Se TV2-innslag her: http://www.tv2.no/v/927503/