Nettside Lytix Biopharma
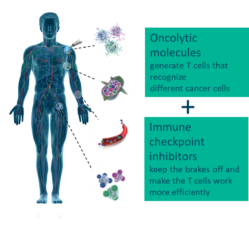
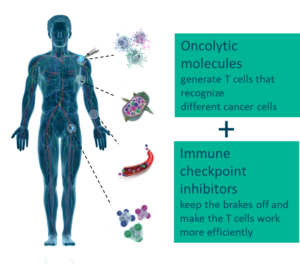
Based in Oslo, Norway, Lytix Biopharma is a clinical stage immuno-oncology company developing novel cancer immunotherapies, an area within cancer therapy that is aimed at activating the patient’s immune system to fight cancer. The Company’s technology is based on pioneering research in “host defense peptides” – nature’s first line of defense towards foreign pathogens. Lytix Biopharma’s lead product, LTX-315, is a first-in-class oncolytic molecule representing a new and superior in situ therapeutic vaccination principle to boost anti-cancer immunity, with the potential to be the ideal combination partner with other types of immunotherapy. LTX-315 target cancer cells and disintegrate their cell membranes, causing immunogenic cell death and release of a patient’s tumor specific antigens. This mode of action allows cytotoxic T cells to recognize, infiltrate, and attack cancer cells. The Company was listed on Euronext Growth in Oslo in June 2021, following a private placement covered by investors such as PBM Capital, a US based, healthcare-focused investment firm.

For one of the most exciting biotechnology companies in Norway, we are looking for an experienced CMC professional.
CMC Manager
Lytix Biopharma is a Norwegian biotech company developing novel oncolytic molecules to fight cancer. Lytix is currently running a Phase II study with its lead compound, LTX-315, for treatment of advanced melanoma in US and EU. Another Phase II study in US is conducted by Lytix` license partner Verrica for treatment of basal cell carcinoma (BCC). LTX-401, a second-generation compound, is in development for entry into human clinical studies for patients with deep-seated lesions.
To follow-up the CMC work (chemistry, manufacture, control) of these and other drug candidates in the research phase, Lytix is seeking an experienced CMC professional to expand and strengthen the team. You will have broad variability in the tasks and extended responsibilities. You will be offered the opportunity to work with a highly dedicated and skilled team which is willing to walk the extra mile.
Main Responsibilities include, but are not limited to:
- Formulation and development activities, manufacture, quality control and release of Drug Substance and Drug Product for use in pre-clinical and clinical studies
- Issuing development reports, CMC documentation etc.
- Selection, qualification and follow-ups of CRO’s, CDMO’s and Consultants as appropriate
- Preparation of regulatory documentation for clinical trial applications
- Development and manufacture that are conducted in accordance with regulatory requirements (EMA/FDA, GMP, ISO, lCH etc) and clinical trial applications (IMPD, IND)
Who are you?
- Msc or PhD in Pharmaceutical Technology, Chemistry, Analytics, Biotechnology or similar
- More than 5 years’ experience from CMC in pharmaceutical or biotech industry
- Good understanding of quality and regulatory requirements
- Experience in managing subcontractors (CDMOs, CROs, consultants) is preferred
- Team player
- Fluent in English, both written and oral
- Highly motivated and results-oriented
- Organized and ability to work independently
The ideal candidate will be a highly motivated individual with experience from, and interest in, working in a small, entrepreneurial environment with broad responsibilities and opportunities.
Additional info:
The position will be based at Lytix Biopharma´s offices in Nydalen, Oslo, Norway.
This position reports to Chief Technical Officer, Gry Stensrud.
Contact information:
The recruitment process is carried out in collaboration with the consulting company Borka Consulting AS.
If you have any questions regarding Lytix Biopharma or the position, feel free to contact our recruitment advisor in Borka Consulting;
Cecilie Borka at +47 928 55 352 or email to cecilie@borka.no.
Please send your application (both motivational letter and CV in PDF format) to cecilie@borka.no.
All inquiries are treated confidentially, also towards Lytix Biopharma, in the initial phase if desired.
Selection will be ongoing: If you think you are the person we ask for, please apply at earliest convenience.
NOTE: Unfortunately, we sometimes experience that applicants do not receive a reply to an email from us, in which case our reply has most likely ended up in the spam filter on your mail server. Please check this if you miss answers from us, we always answer!
Borka Consulting is a specialized search & recruitment company that has 100% focus on competence-based search and recruitment in the Life Sciences sector in Norway, the Nordic countries and in Europe.


Links:
- https://www.healthtalk.no/ltx-315-lytix-biopharma-onkolytisk-virus/lytix-biopharma-sjefen-ser-lyse-tider-for-selskapets-utprovende-kreftmedisiner/169567
- https://medwatch.no/nyheter/legemidler_biotek/article15044446.ece
- https://medwatch.no/nyheter/legemidler_biotek/article14585754.ece
- https://www.finansavisen.no/tema/lytix_biopharma
- https://www.dagensmedisin.no/asco-asco-2022-kongresser/lytix-med-nye-sarkom-data-pa-asco-positivt/298809
- https://soundcloud.com/user-972208711/episode-160