Nettside artbio_inc ARTBIO
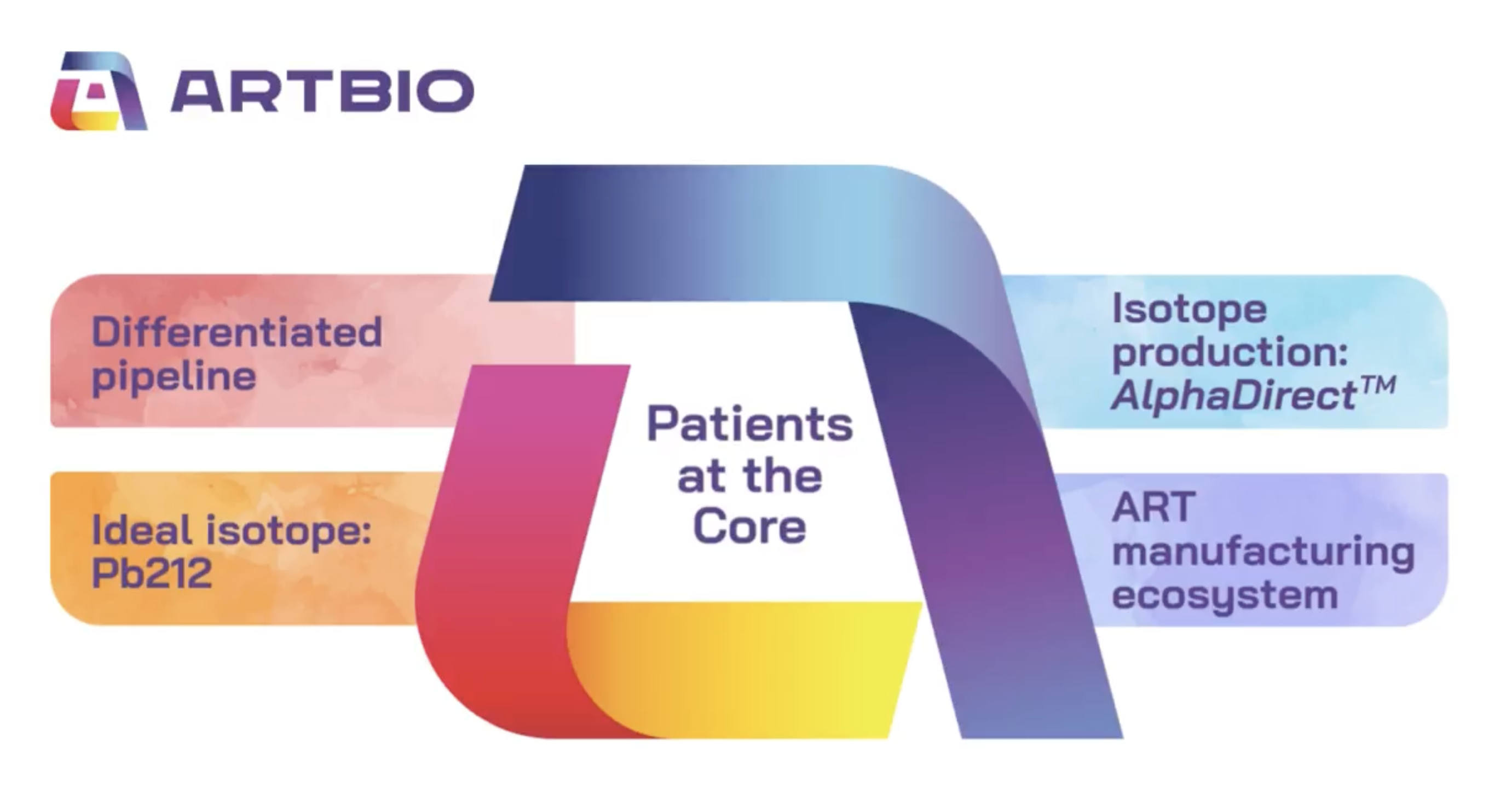
ARTBIO is a clinical-stage radiopharmaceutical biotechnology company focused on developing a new class of alpha radioligand therapies (ART). Our unique patient-centric approach is underpinned by a deep understanding of cancer biology and the infrastructure needed to effectively design, manufacture, and distribute our therapies. More info at www.artbio.com

If you want to continue your career in product design and management in the biotech industry, and you can see yourself part of a strong professional team where you can grow and develop, then join our team!
Product Engineering and Development Lead
✔ clinical-stage radiopharmaceutical biotech ✔ radioligand therapies ✔ targeted cancer therapeutics
- Are you a humble achiever?
- Are you passionate about making a difference for cancer patients
- Are you a humble achiever
- Are you a practical optimist
- Are you a consummate learner and un-learner
- Are you driven by data and cherish transparency
- Are you a compassionate team player
We chose those words very carefully and while we understand that they have an inherent appeal, we ask you to examine them and think about whether you fit our description.
ARTBIO is a clinical-stage radiopharmaceutical biotechnology company with multiple locations, including Oslo, Basel, and soon Cambridge (USA)
Our mission is to develop therapeutics that extend patients’ lives and also improve their quality of life.
We specialize in developing targeted radioligand therapeutics and we are growing our new company with roots in the Oslo ecosystem of radiobiology, and founders with a track record of developing new therapies.
We are backed by international venture capital groups with deep scientific roots who understand what it takes to develop and scale a technology to deliver new therapies.
Join us on an exciting journey in shaping our core technology platform that will power the development of all our therapeutics!
Your primary tasks will be:
We are developing a proprietary radionuclide generator that will be core to the company strategy. You will be part of a distributed team of experts in USA and EU to design the generator, supported by a team of experienced medical device designers, regulatory, manufacturing, supply chain experts.
The first step will be to learn what the team has done. You will need to identify the proper materials to be used in the generator and you will test them according to our use criteria.
Following this extensive design and test phase, you will work with regulatory and technical experts to define the rules to abide by. You will then have to take on the technical design phase, work on user testing, prototyping, and refinement. Ultimately you will ensure that the product lifecycle meets the needs and you will be involved in refining the approaches.
Professional qualifications:
We imagine that you have a technical background within engineering, physical sciences or life sciences with practical experience managing projects and developing new technical products for the primary use by the life science industry. You have chemistry and nuclear chemistry experience with a focus on material properties and engineering.
Experience in radionuclide generators and the radioligand therapeutic space is a plus.
You have extensive professional experience (at least 5 years) in product design and management. You are used to working in a creative environment and can bring structure at the right time and in the right manner when integrating inputs and workstreams from diverse stakeholders to ensure the goals of the project and the product are achieved.
Your communication skills in English are excellent, and you have a well-developed commercial understanding.
Personal qualifications:
You possess great impact, and your professional pride and quality awareness is reflected in your results. You manage complexity and are a fast learner.
As a person, you are humble and self-aware, and always focused on doing the right thing.
The ideal candidate will be a highly motivated individual with interest in working in a small, entrepreneurial environment with broad responsibilities and opportunities.
What we offer:
We offer an informal and flat organization where the distance from thought to action is short, where you can easily make a difference, and where results mean more than titles.
Furthermore, we offer a:
- professional management
- varied and exciting working day in a very competent environment, characterized by interdisciplinary collaboration and short decision paths.
company with big growth ambitions - great degree of autonomy and the opportunity to create your own role
We offer a full-time position, based at our offices in Oslo, Norway or in Basel, Switzerland. The position requires flexibility to travel to work with users and experts in Europe and in US.
Questions and Application process:
This recruitment is carried out in collaboration with the recruitment company Borka Consulting AS.
If you have any questions regarding ARTBIO or the position, feel free to contact our recruitment advisor in Borka Consulting; André Borka at +47 908 31 871.
Please send your application (both motivational letter and CV in PDF format) to andre@borka.no.
All inquiries are treated confidentially, also towards ARTBIO in the initial phase if desired.
Interviews are held regularly, as we want the position filled as soon as possible. However, we are happy to wait for the right candidate.
NOTE: Unfortunately, we sometimes experience that applicants do not receive a reply to an email from us, in which case our reply has most likely ended up in the spam filter on your mail server. Please check this if you miss answers from us, we always answer!
Borka Consulting is a specialized search & recruitment company that has 100% focus on competence-based search and recruitment in the Life Sciences sector in Norway, the Nordic countries and in Europe.