Nettside abfredriksen LinkedIn Facebook Vaccibody
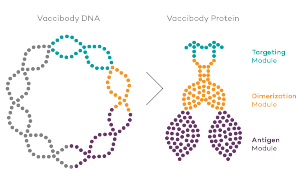
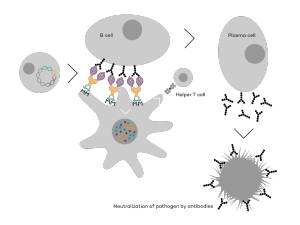
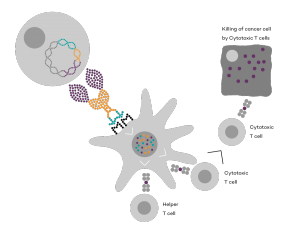
Vaccibody is a clinical-stage biopharmaceutical company dedicated to the discovery and development of novel immunotherapies using the Vaccibody’s proprietary technology to generate best-in-class therapeutics to treat cancers with a high unmet medical need. Vaccibody is a leader in the rapidly developing field of individualized cancer neoantigen vaccines and is currently performing a phase 1/2a clinical trial making novel personalized cancer neoepitope vaccines (VB10.NEO) where one unique vaccine is designed and manufactured for each patient. In addition, Vaccibody's lead drug candidate VB10.16, a therapeutic DNA vaccine against HPV-induced pre-malignancies and malignancies has completed phase 2a showing that VB10.16 vaccination induced rapid, strong and long-lasting immune responses, was well tolerated and showed early signs of clinical effect. A phase 2 study evaluating VB10.16 in combination with atezolizumab (Tecentriq®) in patients with advanced or metastatic cervical cancer is planned to start Q12020
Immune Monitoring Specialist
Unique opportunity to work with immune monitoring of clinical samples in a rapidly growing biotech company developing cutting edge technology for improved cancer therapy.
Vaccibody is looking for an Immune Monitoring Specialist to be responsible for our immune monitoring laboratory. The ideal candidate will be a highly motivated individual with experience from and interest in working in a small, entrepreneurial environment with broad responsibilities and opportunities.
Principal Duties and Responsibilities:
- Develop and perform T cell assays for immune monitoring of clinical samples
- Contribute to establish an immune monitoring lab at Vaccibody for analysis of clinical samples
- Plan, execute and report immune monitoring testing of clinical samples
- Ensure clinical samples are analysed and stored in compliance with GLP, relevant SOPs and regulatory requirement.
- Ensure deliverables meet the agreed quality level
- Write reports and SOPs according to Vaccibody Quality Management System
Required Experience:
- Master/PhD in immunology or other relevant scientific discipline
- A strong understanding of human T cell immunology and cellular assays
- Structured mindset with focus on quality and experienced in QC/QMS from industry
- English fluency is required
- Team player and excellent communicator, proactive and accurate as a person
We can offer challenging responsibilities in a fun and dynamic work environment where you will grow your personal skills through continuous learning and development. At Vaccibody you will collaborate with colleagues who focus on quality and efficiency. The workplace is Forskningsparken in Oslo.
The person will report to Karoline W. Schjetne, VP Scientific Affairs.
For more information, please contact external recruiter:
Cecilie Borka, Borka Consulting at +47 92 85 53 52. Please send your application (both motivational letter and CV) in PDF format to cecilie@borka.no.
Interviews are held consecutively, please show your interest as soon as possible!
All inquiries are treated confidentially, also towards Vaccibody in the initial phase if desired.
———————