Nettside abfredriksen LinkedIn Facebook Vaccibody
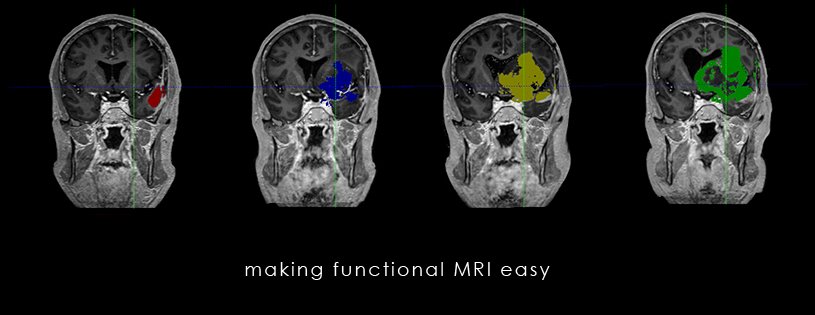
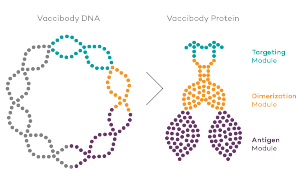
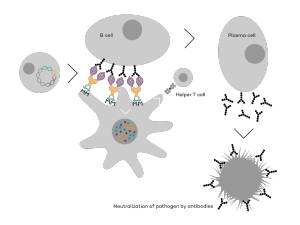
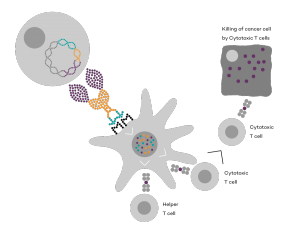
Vaccibody is a clinical-stage biopharmaceutical company dedicated to the discovery and development of novel immunotherapies using the Vaccibody technology to generate best-in-class therapeutics to treat cancers with a high unmet medical need. Vaccibody is a leader in the rapidly developing field of individualized cancer neoantigen vaccines and is and is currently performing a clinical trial making novel personalized cancer neoepitope vaccines where one unique vaccine is designed and manufactured for each patient. In addition, Vaccibody's first program (VB10.16) a therapeutic DNA vaccine against HPV16 induced pre-malignancies and malignancies This first-in-man study evaluates the safety and immunogenicity of VB10.16 in women with high grade cervical intraepithelial neoplasia (HSIL; CIN 2/3 and has now released very promising 6 months data from the phase 2a)
Clinical Study Manager
Amazing opportunity to join a dynamic clinical study team conducting Phase I-III studies
with a unique vaccine technology platform.
Vaccibody is looking for a Clinical Study Manager (CSM) to join their clinical team. The CSM will be responsible for planning, organizing, managing, and controlling the daily operations to ensure progress of the assigned clinical study(ies) meet agreed timelines, costs and quality. The CSM will be the primary liaison to our CRO and vendors, managing all aspects from start-up to close-out activites and ensuring questions answered and issues are resolved. Other responsibilities will include writing/providing input to clinical documentation.
The ideal candidate will be a highly motivated individual with experience from and interest in working in a small, entrepreneurial environment with broad responsibilities and opportunities.
Main Duties and Responsibilities:
- Contribute to the execution of the clinical program according to timelines and cost.
- Ensure CRO oversight and monitor clinical CRO performance, serving as the primary project contact with the CRO/vendor.
- Responsible for planning, set-up and execution of assigned Vaccibody clinical trials according to plan, timeline, resource, and budget.
- Ensure Vaccibody clinical studies are conducted in compliance with GCP, relevant SOPs and regulatory requirements. Ensure the deliverables meet the agreed quality level.
- Contribute in planning, design, write and review clinical protocols/reports for Vaccibody sponsored studies.
- Write input to and review of clinical documentation created in collaboration with CRO or internal team members needed for submissions of Clinical Trial Applications (CTAs/lNDs) to regulatory authorities and Ethics Committees.
The ideal candidate has:
- Master or PhD degree within science
- Minimum 5 years of experience in clinical research
- Study Management experience
- Some global experience from a pharmaceutical company or CRO
- Good knowledge of GCP and regulatory procedures and guidelines
- Cross functional understanding of pharmaceutical research and development
- As a person you are team player and a excellent communicator, both in written and oral form. English fluency (ability to read, write, speak) is required.
The person will report to Mads Axelsen, Chief Medical officer. The workplace is Forskningsparken in Oslo.
For more information, please contact external recruiter:
Cecilie Borka, Borka Consulting at +47 92 85 53 52. Please send your application (both motivational letter and CV) in PDF format to cecilie@borka.no. Interviews are held consecutively, please show your interest as soon as possible!
All inquiries are treated confidentially, also towards Vaccibody in the initial phase if desired
———————