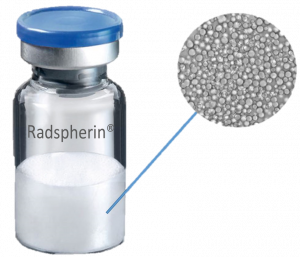
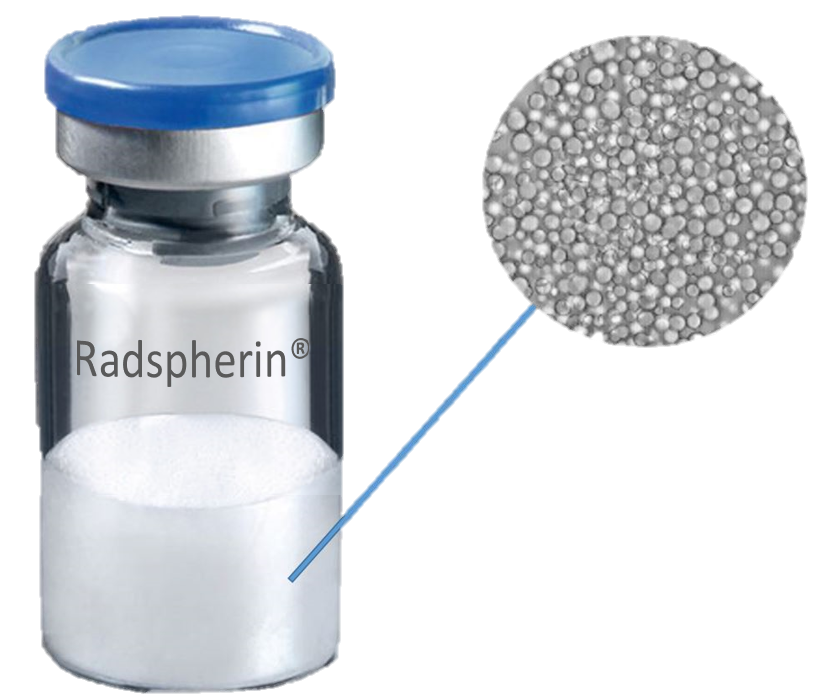
Oncoinvent AS is a privately held Norwegian pharmaceutical company based in Oslo, Norway. The company is committed to developing new innovative products in order to provide better treatment options to cancer patients. The company’s founders started Oncoinvent in 2010 with a view to designing better cancer treatments by applying known physical and chemical principles of selected novel materials in new ways to maximize their medical benefit while minimizing potential safety concerns. This approach has allowed Oncoinvent to explore and develop multiple technological avenues before selecting the company’s first lead product candidate. Oncoinvent has built a Class B production and lab facility for radiopharmaceuticals. The facility received a GMP certificate from the Norwegian Medical Agency in February of 2019. The approval provides the company with the necessary flexibility and capacity for manufacturing clinical trial material for both patients and trial sites, as well as developing the company further. The company’s lead product candicate Radspherin® is a novel alpha-emitting radioactive microsphere suspension designed for treatment of metastatic cancers in body cavities. The radium-224 based therapeutic, Radspherin® has shown strong and consistent anticancer activity at doses being essentially non-toxic in preclinical studies. It is anticipated that the product can potentially treat several forms of metastatic cancer.

Clinical Study Manager (CSM)
✔ expanding biotechnology start-up ✔ First-In-Human trials ✔ oncology ✔ workplace Oslo
Oncoinvent AS is looking to strengthen its team by hiring a Clinical Study Manager at its offices in Oslo, Norway. The position presents a unique opportunity to join an expanding biotechnology start-up that is about to initiate the first-in-human/phase I studies for the lead product candidate, Radspherin®. Radspherin® is a radiopharmaceutical targeting to treat peritoneal carcinomatosis.
The candidates should be looking for the energy and enthusiasm in a start-up company with the ambition to make a difference. Successful candidates should be flexible, team-oriented yet able to work independent, analytical and results oriented.
Responsibilities include:
- Review and input to clinical operations related Standard Operating procedures (SOPs)
- Review and provide input to clinical study protocols and other documents for Oncoinvent sponsored studies
- Overall responsibility for managing day-to-day activities in phase I clinical studies
- Day-to day oversight of study activities to ensure that these are conducted in compliance with ICH/GCP, relevant SOPs and other regulatory requirements and according to plan and budget
- Immediate reporting/communication of deviations and other significant incidences that potentially may have budgetary consequences and impact on project timelines
- Manage Contract Research Organization (CRO) and any other vendor utilized for the phase I studies and act as primary point of contact from sponsor’s side
- Monitor ongoing clinical CRO performance according to Key Performance Indicators (KPIs)
- Accountable for effective management of budgets, timelines for assigned clinical studies and implementation of appropriate standards and processes to ensure clinical study quality
- Manage and lead the day-to-day operations of assigned studies to ensure completion per established project team goals and objectives in compliance with applicable GCP/ICH guidelines and other regulatory requirements
- Contribute to the development of study plans and system set-up; participate in preparation and ensure operational excellence of protocol, eCRF, CSR and other key study team deliverables.
- Track and review study milestones, tasks and timelines
- Review of monitoring visit reports (for Pre-Selection Visits, Site Initiation Visits, Regular Monitoring Visits and Closure Visits), ensuring the CRO meets required timelines for review and submission and ensuring quality sufficient to be inspection ready at all times.
- Oversee the clinical aspects of timely data cleaning, data analysis and the availability of top line results; participate in data reviews and review of statistical analysis plans
- Provides leadership in identifying operational risks and developing mitigation and contingency plans for the phase I studies.
Requirements:
- Minimum 5 years of experience from clinical research, preferably within Oncology
- Minimum 2 years of previous experience within study management
- Master’s degree in Science, Engineering, Pharmacy or similar
- Able to work in Oslo, Norway
- Previous experience in managing multi-sites clinical studies
- Knowledge of ICH GCP
- Demonstrate experience from several areas within clinical research (eg. Data Management and/or Medical Writing) is considered a benefit
- Experience in clinical studies with a radioactive product is considered a benefit
- Fluent in English (Norwegian a benefit)
The workplace is in Nydalen in Oslo, Norway.
The position will report to Helen Blanco, Head Of Clinical Operations at Oncoinvent AS
For more information, please contact external recruiter, Borka Consulting:
Cecilie Borka at +47 928 55 352.
Please send your application (both motivational letter and CV) in PDF format to cecilie@borka.no
Interviews are held consecutively, please show your interest as soon as possible!
All inquiries are treated confidentially, also towards Oncoinvent in the initial phase if desired.