Nettside ABFredriksen Vaccibody
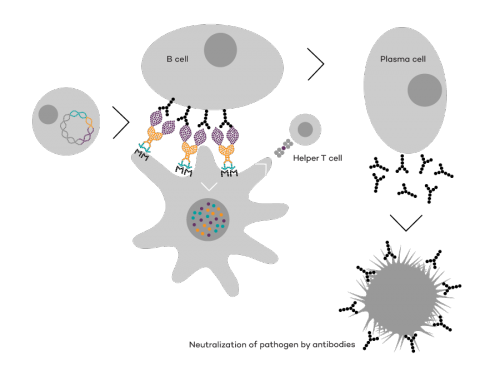
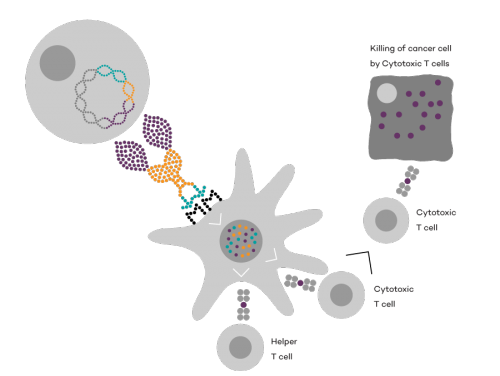
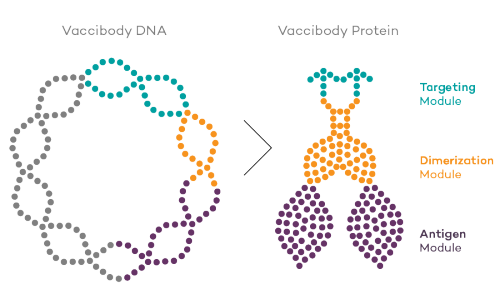
Vaccibody is a leading cancer neoantigen company, which bases it vaccine development on a proprietary patent protected vaccine technology allowing for generation of very efficacious, targeted and safe plasmid DNA vaccines. Vaccibody is looking to enter cancer neoantigen clinical trials within its VB10.NEO program (which implies one cancer vaccine custom-made per patient). The company moreover has recently finalized a clinical phase I trial with VB10.16 (a therapeutic HPV vaccine) and plans to start clinical phase IIa in Q2, 2017

Head of Clinical Operations
Vaccibody is now looking for a leader of its clinical operations. The leader will report to the CEO and will be responsible for all clinical activities for both VB10.NEO and VB10.16 including trial design and execution as well as building up and managing Vaccibody’s internal clinical department alongside its external clinical and regulatory collaboration partners. The ideal candidate will be a highly motivated individual with experience from and interest in working in a small, entrepreneurial environment with broad responsibilities and opportunities.
Principal Duties and Responsibilities
- Assume overall clinical responsibility for the VB10.16 and VB10.NEO programs, including regulatory work and clinical operations
- Lead and guide strategic discussions regarding the clinical trials on VB10.NEO, in particular take responsibility for selection of clinical indications and study design
- Assume responsibility for selecting external collaborators for the clinical trials on VB10.NEO
- Manage and build up a strong Vaccibody clinical team (4-5 people)
- Lead and implement clinical direction in smooth collaboration with rest of the Vaccibody management team
Skills and background
- MD, PhD or MSc in pharmaceutical sciences, biochemistry, biology or related scientific discipline.
- 10+ years of relevant experience in a pharmaceutical or biotech environment
- Strategic and innovative thinker with proven ability to communicate a vision and drive results
- Solid experience in managing clinical operations, preferably from biotech
- Experience with CTA, IND and other relevant filings, including knowledge of relevant EMA and FDA regulations
- Preferably, experience within immuno-oncology/immunotherapy.
- Solution-oriented with strong ability to thrive while operating in the front of a new field with limited regulatory and other guidelines
- Strong ability to identify and resolve critical issues
- Strong ability to solve problems and execute on initiatives.
- Excellent written and verbal communication skills, very strong interpersonal and management skills to collaborate with and direct the work of others (both internal colleagues and external collaborators)
For more information and about the application process please contact external recruiter; Borka Consulting:
André Borka at +47 90831871 (andre@borka.no)
or Cecilie Borka at +47 92855352 (cecilie@borka.no)
Please send your application (motivational letter and CV) in PDF format.
Interviews are held consecutively, so show your interest as soon as possible!
All inquiries are treated confidentially, also towards Vaccibody in the initial phase if desired
Se TV2-innslag her: http://www.tv2.no/v/927503/