Nettside http://twitter.com/ABFredriksen LinkedIn Facebook Vaccibody
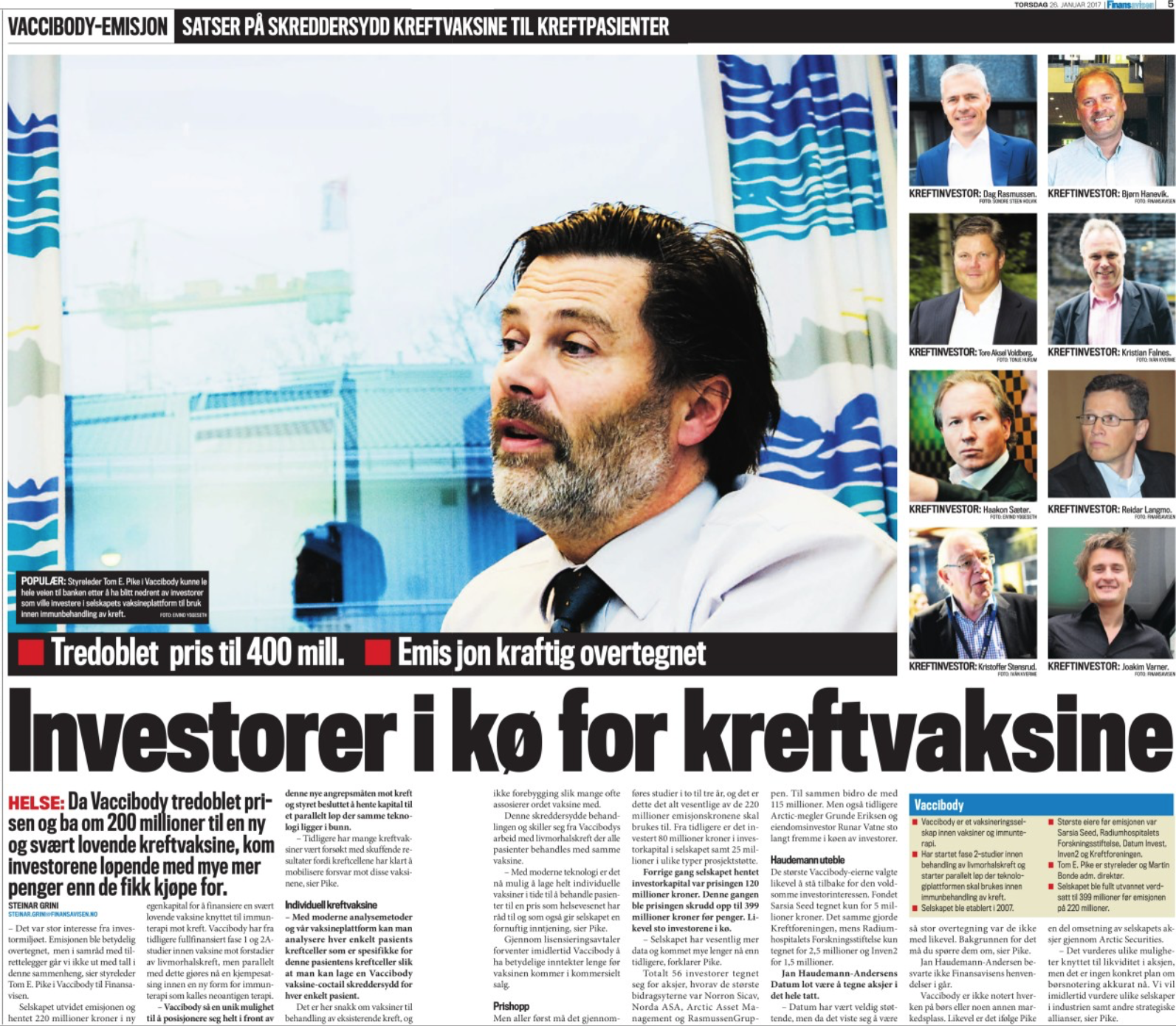
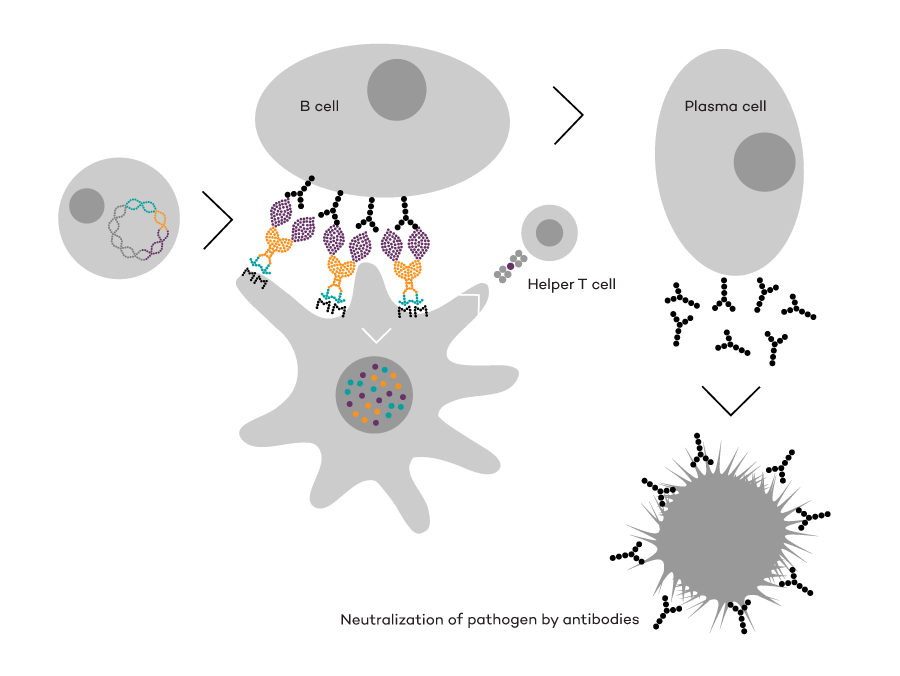
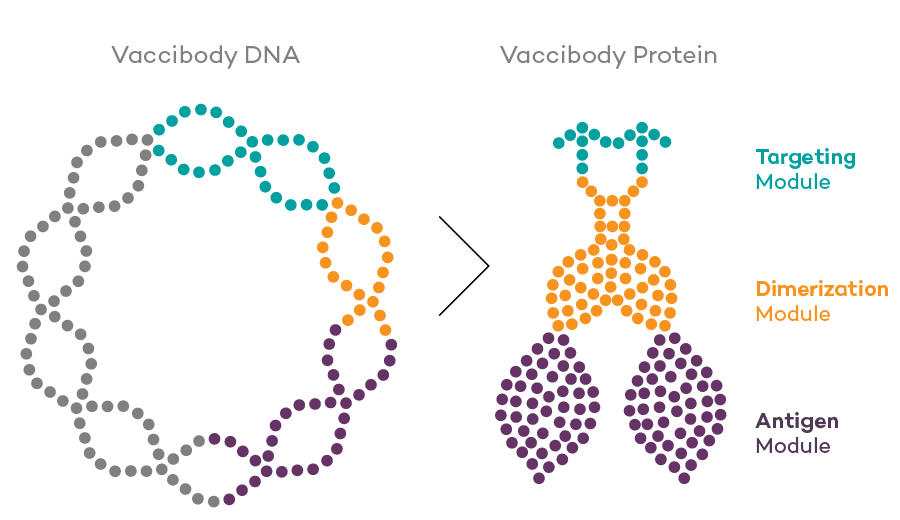
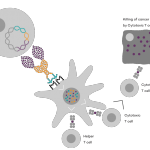
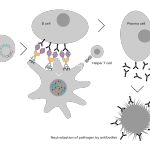
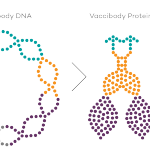
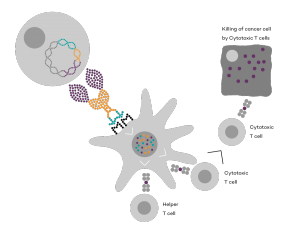
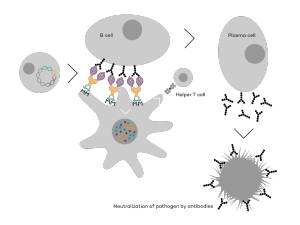
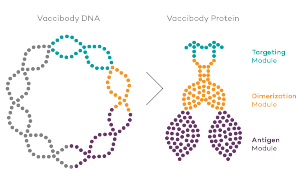
Vaccibody AS is a Norwegian clinical stage immunotherapy company dedicated to the discovery and development of novel immunotherapies. Vaccibody’s front runner program (VB10.16) is a therapeutic DNA vaccine against HPV16 induced precancerous lesions of the cervix (CIN2/3). In a clinical phase I trial, the VB10.16 vaccine has shown excellent safety as well as generation of strong immune responses. The program is now in clinical phase IIa and Vaccibody expects to report 6 months results from the phase IIa by Q3 2018. Vaccibody moreover is a leader in the rapidly developing field of individualized cancer neoantigen vaccines. Vaccibody’s neoantigen vaccine program (VB10.NEO) recently initiated a clinical phase I/IIa trial in patients with locally advanced or metastatic melanoma, non-small cell lung cancer (NSCLC), clear renal cell carcinoma, urothelial cancer or squamous cell carcinoma of head and neck
Director/VP of Business Development
Vaccibody is now looking for a Director/VP of Business Development. He/she will report to the CEO and will be responsible for carrying out business development activities, initially primarily with relation to VB10.16. The ideal candidate will be a highly motivated individual with experience from and interest in working in a small, entrepreneurial environment with broad responsibilities and opportunities.
Principal Duties and Responsibilities:
- Responsibility for business development with relation to VB10.16 with the aim of out-licensing VB10.16 globally, both for treatment of CIN2/3 but also for treatment of HPV16+ cancer, eventually in combination therapy with a checkpoint inhibitor.
- Responsibility for developing and implementing a strategy for out-licensing of VB10.16 globally, both for treatment of CIN2/3 but also for treatment of HPV16+ cancer, eventually in combination therapy with a check pointinhibitor.
- General business development activities, also with relation to Vaccibody’s VB10.NEO program and potential new programs.
Skills and background
- PhD or MSc in pharmaceutical sciences, biochemistry, biology, chemical engineering, or related scientific discipline
- 5+ years of relevant experience in a pharmaceutical or biotech business development environment
- Solution-oriented with strong ability to thrive while operating with high level of uncertainty
- Strong ability to identify and resolve critical issues
- Excellent written and verbal communication skills, very strong interpersonal skills and highly developed skills to collaborate with others (both internal colleagues and external potential partners).
The workplace is Forskningsparken in Oslo
For more information, please contact external recruiter:
Cecilie Borka, Borka Consulting at +47 92 85 53 52. Please send your application (both motivational letter and CV) in PDF format to cecilie@borka.no.
Interviews are held consecutively, so show your interest as soon as possible!
All inquiries are treated confidentially, also towards Vaccibody in the initial phase if desired
.